





Related Attributes
Product details
AZD-9291 mesylate is indicated for the treatment of patients with advanced non-small cell lung cancer with epidermal growth factor receptor (EGFR)T790M mutations or resistance to other EGFR inhibitors. The EGFR inhibitor, ocimotinib, must first be tested for T790M mutation before clinical use. Only patients with T790M mutations in non-small cell lung cancer can be targeted with ocimotinib.
Uses of Osimertinib Mesylate.
AZD-9291 mesylate is primarily used in the treatment of lung cancer, especially in patients with EGFR(epidermal growth factor receptor) mutation-positive non-small cell lung cancer. It selectively inhibits EGFR mutated proteins, and shows high selectivity and effectiveness against T790M secondary mutated EGFR, which has a good clinical application prospect.

Preparation of Osimertinib Mesylate.
With 4 - fluoro - 2 - methoxy - 5 - nitroaniline with 3 - (2 - chloro pyrimidine - 4 - base) - 1 - methyl indole reaction under the catalysis of monohydrate for N - (4 - fluoro - 2 - methoxy - 5 - nitrobenzene) - 4 - (1 - methyl - 1 h - indole - 3 -) pyrimidine - 2 - amine, after using N, N, N '- trimethyl ethylenediamine instead of fluorine, The nitro group was reduced with ferric ammonium and then reacted with 3-chloropropionyl chloride and then eliminated to obtain AZD9291 with a yield of 17.5%. AZD9291(mesylate) was prepared by salting AZD9291 with mesylate.
Product Method of Bulk Osimertinib Mesylate Powder.
WHY CHOOES US?

OUR CERTIFICATE

CUSTOM PROCESS

OUR PACKAGE

OUR EXHIBITION

OUR FACTORY

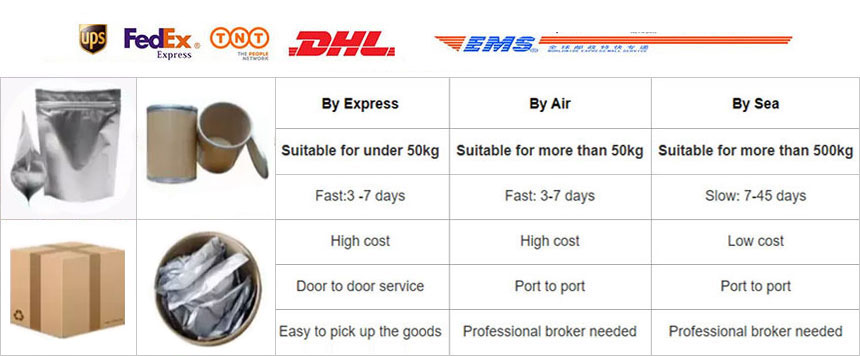
Shipping
Pharmaceutical Intermediate manufacturers
©2022 Xi'an Henrikang Biotech Co., Ltd.,