





Related Attributes
Product details
Chloramphenicol acts by inhibiting bacterial protein synthesis. It binds reversibly to the 50S subunit on the bacterial 70S ribosome, thereby preventing the amino acid terminus of the aminoacyl tRNA from binding to the receptor on the ribosome. This prevents the amino acid substrate from interacting with transpeptidases and forming peptide bonds (see Pratt and Fekety, 1986).
Chloramphenicol is normally a bacteriostatic agent, but therapeutic concentrations can also be bactericidal against common meningeal pathogens such as H. influenzae, S. meningitidis and S. pneumoniae (Rahal and Simberkoff, 1979). The 70S ribosomes in the mitochondria of mammals have physicochemical properties similar to those of the drug in bacterial cells.
Many of the adverse effects of chloramphenicol, including dose-related myelosuppression and grey syndrome, appear to be due to inhibition of host mitochondrial protein synthesis.
Chloramphenicol is used primarily for the treatment of urinary tract infections, pneumonia, abdominal infections, and sepsis caused by sensitive bacteria, as well as topical eye and ear drops. However, because of its effect on the haematopoietic system, it is no longer the drug of choice.

Uses of Chloramphenicol.
It is a broad-spectrum antibiotic, the first choice for the treatment of typhoid fever, paratyphoid fever, one of the most effective drugs for the treatment of anaerobic infections, and is also used for the treatment of various infectious diseases caused by sensitive microorganisms. Due to the serious adverse reactions now used less and less
Used for the treatment of infections caused by typhoid, dysentery, E. coli, influenza, Brucella, pneumococcus and so on.
Antibacterial spectrum, action and use are the same as chloramphenicol..
Drug interactions of Chloramphenicol.
Chloramphenicol inhibits hepatic microsomal enzymes in the metabolism of phenytoin, toluene sulfobutylurea (methylsulfonylurea), chlorosulfopropylurea, and dicoumarin (and possibly other drugs), resulting in a prolonged half-life in vivo and an increase in serum concentrations. Exacerbation of poisoning to the point of death has been reported.
On the other hand, phenobarbital, phenytoin and rifampicin (Prober, 1985) reduce serum chloramphenicol concentrations, presumably due to the induction of hepatic enzymes by the drugs. Therefore, serum chloramphenicol concentrations should be monitored if concomitant drugs that may affect the pharmacokinetic profile of chloramphenicol are used. Chloramphenicol delays the response of iron, folic acid and vitamin B12 to anaemia treatment. It also
interferes with the host recall response to tetanus toxoid. Therefore, the concomitant application of chloramphenicol with active immunisers should probably also be avoided. The clinical significance of chloramphenicol's antagonistic effect on the bactericidal action of penicillin remains unclear, although it has been demonstrated in vitro and in animal experiments. Such combinations should be used only when the benefits of such treatment have been demonstrated.
Production Method of Chloramphenicol.
The production methods of chloramphenicol have been researched a lot in different countries of the world, which are summarised as follows: (1) p-nitroacetophenone method; (2) styrene method; (3) cinnamyl alcohol method; (4) p-nitrocinnamyl alcohol method; (5) p-nitrobenzaldehyde method. In China, the p-nitroacetophenone method is used, which obtains chloramphenicol from ethylbenzene by nitration, oxidation, bromination, salting, hydrolysis, acetylation, addition, reduction, decomposition, splitting and dichloroacetylation.
WHY CHOOES US?

OUR CERTIFICATE

CUSTOM PROCESS

OUR PACKAGE

OUR EXHIBITION

OUR FACTORY

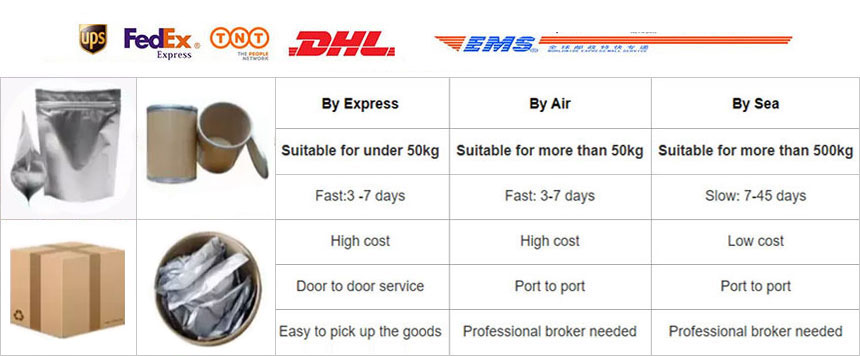
Shipping
Pharmaceutical Intermediate manufacturers
©2022 Xi'an Henrikang Biotech Co., Ltd.,