





Related Attributes
Product details
Pefloxacin is a third-generation quinolone with a 4-quinone nucleus developed by Roger Bellon Pharmaceuticals, France, and was marketed in 1985, with the chemical name of 1-ethyl-6-fluoro-1,4-dihydro-7-(4-methylpiperazin-1-yl)-4-oxo-3-quinolinecarboxylic acid.
It has a wide antibacterial spectrum and strong antibacterial activity, and its mode of action is to inhibit bacterial DNA protease. Multidrug-resistant bacteria (including nosocomial strains) are not cross-resistant to Pefloxacin.
The efficacy of Pefloxacin on certain serious infections is comparable or superior to that of third and fourth generation cephalosporins, new aminoglycoside antibiotics and ciprofloxacin.
Pefloxacin currently occupies an important position in the antimicrobial therapy of various septic infectious diseases, especially central nervous system infections, cardiovascular infections, respiratory infections, gynaecological infections and bone infections.

Uses of Pefloxacin mesylate.
Various infections caused by pefloxacin-susceptible organisms: urinary tract infections; respiratory tract infections; ear, nose and throat infections; gynaecological and reproductive system infections; abdominal and hepatic and biliary system infections; bone and joint infections; skin infections; sepsis and endocarditis; meningitis.
Because serious adverse reactions have been reported with the use of fluoroquinolones, including pefloxacin mesylate for injection, and because in some patients, acute bacterial sinusitis, acute exacerbation of chronic bronchitis, simple urinary tract infections, and acute uncomplicated cystitis are self-limiting, pefloxacin mesylate for injection should be used only in the absence of other drug therapy.

Pharmacological Effect of Pefloxacin mesylate.
1. Pefloxacin mesylate is a quinolone antibacterial drug with broad-spectrum antimicrobial effect, which has good antibacterial effect on the following bacteria: most of the bacteria in the family of Enterobacteriaceae, including Escherichia coli, Klebsiella, Aspergillus, Shigella, typhoid fever and Salmonella, as well as Haemophilus influenzae, Neisseria, etc. It also has a certain antibacterial effect on Pseudomonas aeruginosa and Staphylococcus aureus.
Pseudomonas aeruginosa and Staphylococcus aureus also have a certain antibacterial effect. It has only mild effect on pneumococcus, various groups of streptococcus and enterococcus. In addition, it also has antibacterial activity against leprosy bacillus.
2. Pefloxacin mesylate is a bactericide, which inhibits the synthesis and replication of bacterial DNA by acting on the A subunit of bacterial DNA helicase, leading to bacterial death.

Drug interactions of Bulk Pefloxacin mesylate Powder.
1, Urinary alkalinising agents may reduce the solubility of the product in the urine, leading to crystalluria and nephrotoxicity.
2, This product and theophyllines when used in combination may be due to competitive inhibition with the cytochrome P450 binding site. This may lead to significant reduction of hepatic elimination of theophyllines, prolongation of plasma elimination half-life (t1/2β), increase in blood concentration, symptoms of theophylline toxicity, such as nausea, vomiting, tremor, restlessness, agitation, convulsions, palpitations, etc., and should be avoided, and when it can't be avoided, theophylline blood concentration should be measured and the dose should be adjusted.
3, cyclosporine and this product when used in combination, its blood concentration increased, must monitor cyclosporine blood concentration, and adjust the dose.
4, this product and anticoagulant warfarin when used together can enhance the latter's anticoagulant effect. When used together, the patient's prothrombin time should be closely monitored and the dose should be adjusted.
5, probenecid can reduce the product from the renal tubular secretion of 50% of the drug, when used in combination can be due to the increase in blood concentration of the product and toxicity.
6, this product interferes with the metabolism of caffeine, thus leading to reduced caffeine elimination, blood elimination half-life (t1/2β) prolongation. And may produce central nervous system toxicity, the combination should be closely monitor the patient's blood concentration of caffeine, and adjust the dose.
WHY CHOOES US?

OUR CERTIFICATE

CUSTOM PROCESS

OUR PACKAGE

OUR EXHIBITION

OUR FACTORY

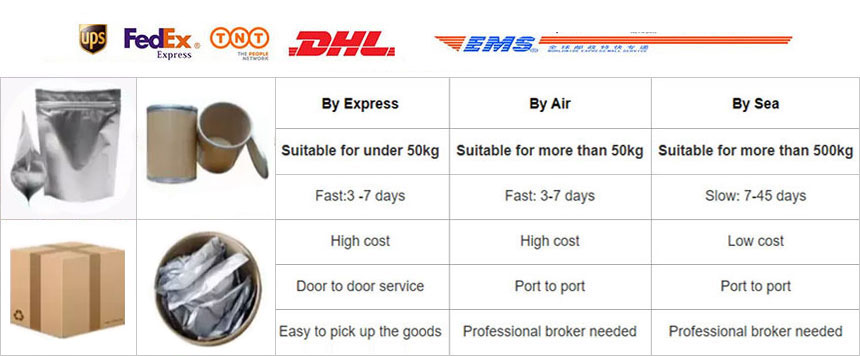
Shipping
Pharmaceutical Intermediate manufacturers
©2022 Xi'an Henrikang Biotech Co., Ltd.,