





Related Attributes
Product details
Application and synthesis of Pregelatinized Starch Excipient (medicinal excipients) :
The usual dosage and maximum dosage were 2560mg orally, 25.3mg/g for general external use, and 39mg for rectal or urethral administration. [1] According to the IIG [2] : Pregummed starch: Route of administration; Dosage form maximum oral dosage; Capsule 600.00MG orally; Hard (gelatin) capsules (quick/full release)70.00MG orally; Coated soft (gelatin) capsule 15.00MG orally; Hard (gelatin) capsules 128.75MG orally; Continuous action capsules 141.75MG orally; Drops 1.20% orally; Suspension 1.50% oral; Continuous acting suspension 12.50% oral; 345.95MG tablet orally; Coated tablets (rapid/complete release)125.00MG orally; Film coated tablets (rapid/complete release)71.35MG orally; Uncoated chewable tablets (rapid/complete release)32.00MG orally; Coated tablet 73.00MG orally; Controlled release tablet 86.94MG orally; Delayed release enteric coated tablet 64.80MG orally; Sustained release tablet 252.80MG orally; Film coating tablet 240.00MG orally; Tangyi tablet 9.40MG orally; Continuous acting tablet 60.00MG orally; Continuous acting coating tablet 33.75MG orally; Continuous acting film coated tablet 75.00MG orally; Uncoated rhombus tablet 80.00MG oral -21; Tablets 22.25MG oral -21; Coated tablet 6.60MG oral -28; Tablets 26.35MG oral -28; Coated tablet 6.60MG sublingual administration route; Tablets 43.00MG corn pre-gummed starch: route of administration; Dosage form maximum oral dosage; Capsule 195.90MG orally; Coated soft (gelatin) capsule 27.75MG oral; Tablet 482.00MG orally; Coating tablet 26.40MG orally; Membrane-coated tablet 70.00MG vaginal administration; Insert 210.00MG pregummed tapioca starch: route of administration; Dosage form maximum oral dosage; Capsule 100.00MG orally; Tablets 5.00MG[1] Japan Association of Pharmaceutical Additives. Japan Book of Pharmaceutical Additives.2007 edition [S]
Stability and storage conditions of Pregelatinized Starches:
Pregelatinized Starches is stable but hygroscopic and should be kept in an airtight container. Put in a cool, dry place [1] [1] RaymondCRowe, PaulJSheskeyandSianCOwen. The Hand book of Pharmaceutical Excipients.FourthEdition.[M]London,Chicago:Pharmaceutical Press.
Pregelatinized Starches function:
Thinner for tablets and capsules; Disintegrators for tablets and capsules; A binder for tablets. Pregelatinized starch is modified starch, used in oral capsules, tablets prescription, commonly used as a binder, thinner and disintegrating agent. Compared with starch, the pre-gummed starch can increase fluidity and compressibility, and is used as a binder in the dry pressing process. Among them, the pre-gummed starch plays the role of self-lubricant. When used with other excipients, it is also necessary to add lubricant, generally adding 0.25% (W/W) magnesium stearate. Too much dosage is bad for the strength and dissolution of tablets. Therefore, it is generally best to lubricate pre-gummed starch with stearic acid. Pre-gummed starch can also be used for wet granulation.
WHY CHOOES US?

OUR CERTIFICATE

CUSTOM PROCESS

OUR PACKAGE

OUR EXHIBITION

OUR FACTORY

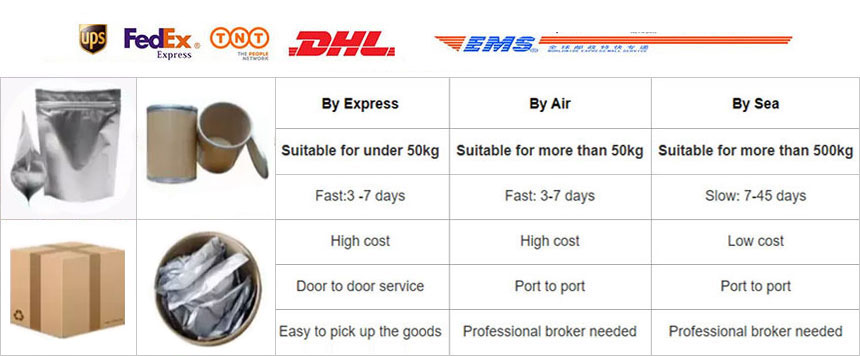
Shipping
Pharmaceutical Intermediate manufacturers
©2022 Xi'an Henrikang Biotech Co., Ltd.,