





Related Attributes
Product details
Sodium chloride is an important chemical raw material for the preparation of chlorine, sodium metal, caustic soda, soda ash and other substances, and is widely used in dyestuffs, ceramics, metallurgy, leather, soap, refrigeration and so on. In analytical chemistry, sodium chloride is a reagent for the determination of fluorine and silicates, and a benchmark reagent for the calibration of silver nitrate.
Sodium chloride is an important substrate to maintain the volume of extracellular fluid, also has a certain role in regulating the acid-base balance of body fluids, or to maintain the neuromuscular stress is one of the important factors.
It is mainly used for the prevention and treatment of low sodium syndrome, sodium deficiency dehydration (such as burns, diarrhea, shock, etc.), heat stroke, etc.; externally used for washing the eyes, nose, wounds, etc.; 10% hypertonic sodium chloride solution can be injected intravenously to promote gastrointestinal peristalsis and enhance digestive function.

Uses of Sodium Chloride.
Sodium chloride is used in the manufacture of soda ash and caustic soda and other chemical raw materials, used in ore smelting, food industry and fishery for salt pickling, also used as raw material for seasoning and refined salt, used as electrolyte and acid-base balance regulator, salt precipitant and diluent of tablets, capsules, etc., used as an analytical reagent, and also used in the preparation of biological culture medium.

Preparation of Sodium Chloride.
A method for producing sodium chloride, characterized in that it comprises the following steps:
(1) preparing a brine comprising at least 150 g/l sodium chloride by dissolving a source of sodium chloride in water;
(2) Cooling the resulting brine to a temperature below 0°C but above the eutectic temperature of the resulting brine by indirect cooling in a self-cleaning fluidized bed heat exchanger/crystallizer to form a slurry and masterbatch comprising sodium chloride dihydrate, said brine not comprising any solid sodium chloride as judged by the naked eye;
(3) Separating the sodium chloride dihydrate formed in step (2) at the eutectic temperature such that a stream rich in sodium chloride dihydrate is formed;
(4) feeding the sodium chloride dihydrate into a recrystallizer to form sodium chloride and a mother liquor.
Product Method of Bulk Sodium Chloride Powder.
WHY CHOOES US?

OUR CERTIFICATE

CUSTOM PROCESS

OUR PACKAGE

OUR EXHIBITION

OUR FACTORY

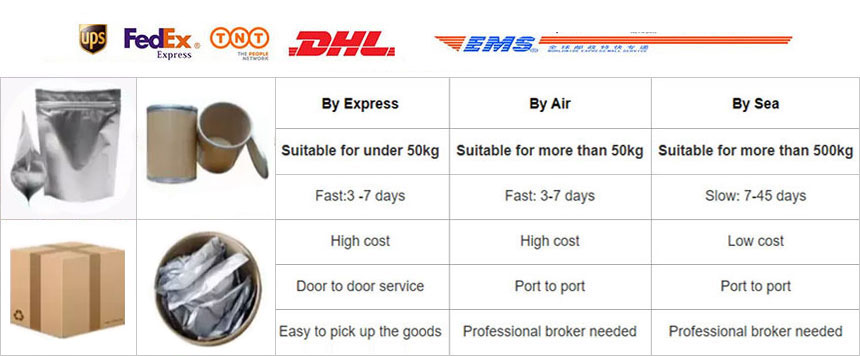
Shipping
Pharmaceutical Intermediate manufacturers
©2022 Xi'an Henrikang Biotech Co., Ltd.,