







Related Attributes
Product details
Lapatinib, also known as Tykerb, is an oral reversible small molecule tyrosine kinase inhibitor targeting HER-1/HER-2. Lapatinib has a different mechanism of action than Herceptin, an approved humanized monoclonal antibody drug, and is able to downregulate cell proliferation signaling by dual blockade of the HER-1/HER-2 pathway.
The clinical indication is for the combination of capecitabine for the treatment of advanced or metastatic breast cancer with HER2 overexpression and prior treatment including anthracycline, paclitaxel and trastuzumab. The efficiency of monotherapy in the first line is about 12-24%, and general clinical guidelines recommend second-line use. The efficiency of combination therapy with capecitabine is about 26-33%, with a clinical benefit rate of up to 71.3%.

Application/Function of Lapatinib Powder .
Lapatinib in combination with capecitabine is indicated for the treatment of patients with advanced or metastatic breast cancer with HER2 (ErbB-2 overexpression) who have received prior therapy including anthracycline, paclitaxel, or trastuzumab.
Lapatinib, a new drug for targeted breast cancer therapy, is a tyrosine kinase inhibitor that effectively inhibits human epidermal growth factor receptor-1 (ErbB1) and human epidermal growth factor receptor-2 (ErbB2) tyrosine kinase activity.
It is unique in that it can act through multiple pathways to prevent breast cancer cells from receiving the signals they need to grow. The mechanism of action is to inhibit the intracellular ATP sites of EGFR (ErbB-1) and HER2 (ErbB-2) to block tumor cell phosphorylation and activation, and to block down-regulated signaling through homo- and heterodimerization of EGFR (ErbB-1) and HER2 (ErbB-1).

Production method of Lapatinib Powder .
In vitro lapatinib tablets inhibit CYP3A4 and CYP2C8 at therapeutic concentrations and are metabolized primarily by CYP3A4. Drugs that inhibit this enzyme activity significantly increase the blood concentration of lapatinib. Ketoconazole, 0.2 g twice/d per dose, increased lapatinib AUC 3-7-fold and prolonged half-life 1.7-fold after 7 d. Oral CYP3A4 inducer at 100 mg twice daily in healthy volunteers was changed to 200 mg twice daily after 3 d for a total of 17 d. Lapatinib AUC was reduced by 72%.
Lapatinib is a transporter of P-glycoprotein, and drugs that inhibit glycoprotein may increase the blood concentration of the drug.

Lapatinib mesylate tablets are small molecule 4-anilinoquinazoline-like receptor tyrosine kinase inhibitors that inhibit epidermal growth factor receptor (ErbB1) and human epidermal factor receptor 2 (ErbB2). four breast cancer cell lines, BT474 and SKBr3, were sensitive to lapatinib with semi-inhibitory concentrations of 25 and 32 nmol/L, while MDA-MB-468 and T47D cell lines were not sensitive, with semi-inhibitory concentrations at the micromolar level level, and for 2 cell lines of bladder cancer,
RT112 (high expression of ErbB1 and ErbB2) and J82 (low expression of ErbB1 and ErbB2), enhanced the efficacy of cisplatin. It inhibited epidermal factor-driven tumor growth in a variety of animals. Lapatinib is effective against trastuzumab-resistant tumor cell lines.
Adverse reactions to Lapatinib Powder:
The most common adverse reactions to Lapatinib are gastrointestinal reactions, i.e., nausea, vomiting, and diarrhea. In addition, skin reactions, such as rash, may occur. The adverse reactions are relatively mild and serious adverse reactions, such as cardiotoxicity and interstitial lung disease/pneumonia, are extremely rare. It is important to evaluate the left ventricular ejection fraction before taking lapatinib and to test it continuously during the course of the drug to prevent cardiotoxicity. If abnormal left ventricular ejection fraction occurs, the drug should be administered or discontinued under medical supervision.
WHY CHOOES US?

OUR CERTIFICATE

CUSTOM PROCESS

OUR PACKAGE

OUR EXHIBITION

OUR FACTORY

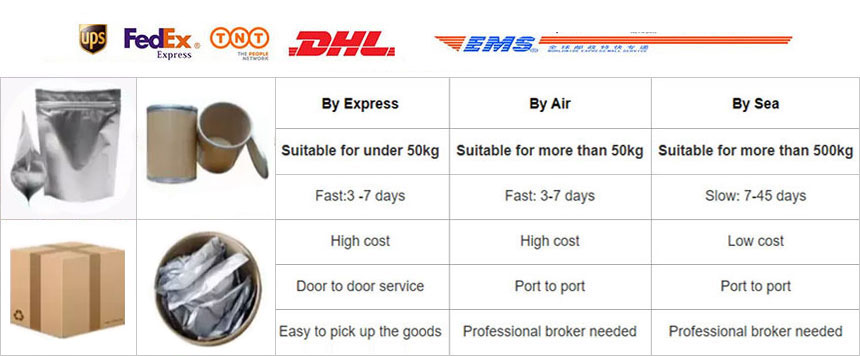
Shipping
Pharmaceutical Intermediate manufacturers
©2022 Xi'an Henrikang Biotech Co., Ltd.,